In gene therapy development, optimizing the production of delivery vehicles and genetic payloads is a crucial aspect. In contrast to monoclonal antibodies and other biologics, the characterization and formulation of delivery vectors, such as adeno-associated viruses (AAVs) and lipid nanoparticles (LNPs), are still considered fresh areas of development.
The production of these molecules is difficult with various requirements to optimize and scale up.
Desalting or buffer exchange for nucleic acid payloads is a basic procedure in sample preparation.
LNPs are often synthesized by combining lipids in an organic solvent solution with the aqueous drug-containing solution. After the formation of LNPs, solvents like ethanol should be removed immediately to ensure LNP integrity and drug encapsulation.
AAVs are frequently cleaned and concentrated using ultrafiltration devices. However, it can be labor-intensive and challenging to manage large sample numbers. Dialysis is generally a preferred gentle technique, but takes more time and needs a secondary concentration step to decrease the sample volume.
All gene therapy-related molecules need robust buffer exchange and preparation techniques, which will not compromise the integrity of the sample while ensuring high recovery rates.
Existing exchange techniques are slow and demand a high level of manual time and work. These are also not sufficient for scale up or an efficient analytical characterization method.
Big Tuna features enhanced processes customized for each sample type. This automates the process to increase sample throughput and ensure sample integrity.
Big Tuna has been built to automate low-volume, high throughput buffer exchange and sample cleanup (Figure 1). It employs a pressure-based ultra-filtration/diafiltration (UF/DF) technique to remove and replace the buffer.
Figure 1. Big Tuna automates buffer exchange for up to 96 unique samples with Unfilter 96 or up to 24 unique samples with Unfilter 24. Image Credit: Unchained Labs
The plate is gently mixed during the pressure-based filtration process to ensure that the samples flow uniformly and do not accumulate at the membrane surface, which simultaneously is faster and more gentle than dead-end filtration techniques.
Buffer exchange using Big Tuna is a highly adaptable and customizable process, facilitating buffer exchange of up to 96 or 24 unique samples and formulations in one run. Unchained Labs developed two filter plate formats for this process, the Unfilters, as depicted in Figure 2.
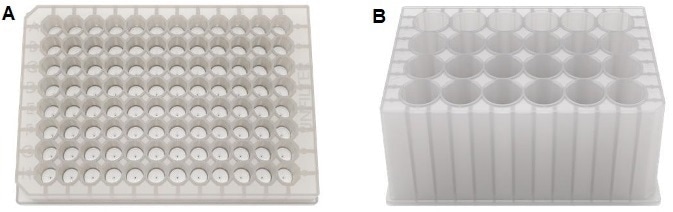
Figure 2. Big Tuna can accommodate both Unfilter 96 and Unfilter 24. A: Unfilter 96 allows for up to 96 samples to be buffer exchanged simultaneously at volumes of 100–450 μL per well. B: Unfilter 24 allows for up to 24 samples to be buffer exchanged simultaneously at volumes of 0.45–8 mL per well. Image Credit: Unchained Labs
Unfilter 96 can process up to 96 samples ranging from 100 to 450 μL, and Unfilter 24 is capable of processing 24 samples ranging from 0.45 to 8 mL in a single experiment. Prior to the run, the Unfilter 96 or Unfilter 24 is filled with the sample to be exchanged, and positioned in the exchange chamber.
The new buffer is positioned on the deck. On executing the run, Big Tuna alternates between volume measurement, filtration and new buffer addition to buffer exchange samples (Figure 3).
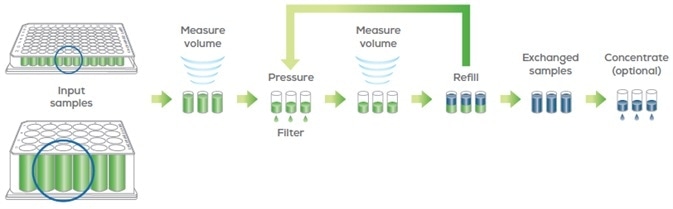
Figure 3. Big Tuna uses a pressure-based UF/DF method with gentle orbital mixing to buffer exchange proteins with the Unfilter 96 or Unfilter 24. Image Credit: Unchained Labs
Big Tuna is loaded with three user-selectable applications, which are designed to serve different processing requirements. The Buffer Exchange application is capable of automating the buffer exchange process with the choice of additionally concentrating the sample after the exchange is complete.
The Concentrate Only mode concentrates sample to a new target volume without requiring buffer exchange. Finally, the Reduce Sample Volume application concentrates up to 24 dilute samples in parallel from ≤48 mL to 8 mL. It works similarly to buffer exchange, but adds dilute sample instead of buffer.
It is possible to run each method independently or consecutively to yield the desired results.
This article describes the usage and key applications of Big Tuna in gene therapy and vaccine sample preparation and purification using particular presets with optimized parameters for each type of molecule.
In the case of LNPs, ethanol will be removed as part of the exchange process. Diluted and large volume AAVs will be concentrated into manageable volumes. Lastly, the DNA is cleaned to remove any salt content and EDTA to prepare for storage or downstream applications.
Methods
LNP buffer exchange and concentration
Firefly Luciferase mRNA encapsulated LNPs (Fluc mRNA-LNPs) were provided by Precision NanoSystems Inc. (Vancouver, BC). Fluc mRNA-LNP stock in PBS measuring 78.4 μg/mL was diluted three-fold into 10% EtOH in PBS. Two wells of a 100 kDa Unfilter 24 were filled with 1345 μL of the diluted LNP.
Table 1 illustrates the important buffer exchange parameters. The LNP preset exchange parameters were used for the experiment. The buffer exchange protocol was set to 99% total exchange per well at a target volume removal of 50% at 60 psi per cycle.
The final well volume was targeted at 450 μL to achieve the final concentration of 78.4 μg/mL.
Table 1. Key buffer exchange parameters used for LNP buffer exchange and concentration performed in a 100 kDa Unfilter 24. Wells were run in duplicate. Source: Unchained Labs
| Parameter | LNP settings |
|---|---|
| Target exchange % | 99% |
| Target volume removed per cycle | 50% |
| Initial LNP concentration | 26.1 µg/mL |
| Initial well volume | 1345 µL |
| Target final concentration | 78.4 µg/mL |
| Target final well volume | 450 µL |
Experimental design and operation were performed using Big Tuna Client. The software reported the total processing time, initial and final well volumes and final percent exchange.
RiboGreen® (from ThermoFisher) was used to quantify the encapsulation efficiency before and after an exchange with a SpectraMax i3 plate reader (from Molecular Devices).
AAV buffer exchange and concentration
An AAV sample of 9.4E13 cp/mL AAV9-CMV-GFP (from Virovek) to 5E12 cp/mL was nominally diluted in PBS, pH 7.0, with 0.001% Pluronic F-68. Diluted AAVs were pipetted manually into a 30 kDa Unfilter 96 or Unfilter 24.
Then, the AAVs were exchanged into PBS, pH 7.0, with 0.001% Pluronic F-68 and then concentrated three-fold.
Table 2 highlights important experiment setup and parameters. AAV preset exchange parameters were utilized in the experiment. The buffer exchange protocol was set to 96% total exchange per well with a target removal of 33% per well at 15 psi.
Table 2. Key buffer exchange parameters used for AAV buffer exchange and concentration in 30 kDa Unfilter 96 and Unfilter 24. Wells were run in triplicate. Source: Unchained Labs
| Parameter | AAV settings | |
|---|---|---|
| Unfilter 96 | Unfilter 24 | |
| Target exchange % | 96% | 96% |
| Target volume removed per cycle | 33% | 33% |
| Initial AAV conc. (cp/mL) | 7.89E12 | 8.17E12 |
| Initial well volume | 450 µL | 1.5 mL |
| Target final conc. (cp/mL) | 2.38E13 | 2.45E13 |
| Target final well volume (µL) | 150 | 500 |
Before and after the exchange and concentration procedure, the Stunner AAV Quant application (from Unchained Labs) was employed to analyze concentration and capsid empty/full ratio.
Dilute AAV: Reduce sample volume
AAV9-CMV-GFP (Virovek) was nominally diluted with 0.001% Pluronic F-68 to 5E11 cp/mL in PBS, pH 7.0. The diluted AAV9 measuring 8 mL was pipetted manually into three wells of a 30 kDa Unfilter 24. The AAV9 stock measuring 10 mL was pipetted manually into three wells of a 4×6 reservoir.
A two-step procedure was performed to reduce the diluted AAV from 18 mL to a final volume of 500 µL. Initially, 18 mL of dilute AAV9 was reduced to 8 mL using the Reduce Sample Volume application on Big Tuna Client.
A Concentrate Only experiment with the same Unfilter 24 plate concentrated each well to 500 µL, for a total 36-fold concentration from the 18 mL stock.
Table 3 presents the important experiment setup and parameters. AAV preset exchange parameters were utilized in both steps.
Table 3. Targeted initial and final concentration and volume used to concentrate dilute AAV up to 36-fold in a 30 kDa Unfilter 24. Wells were run in triplicate. Source: Unchained Labs
| Parameter | AAV settings |
|---|---|
| Initial AAV concentration | 5E11 cp/mL |
| Initial volume | 18 mL |
| Target final concentration | 1.8E13 cp/mL |
| Target final well volume | 500 µL |
The concentration and capsid empty/full ratio were analyzed before and after the concentration steps using the Stunner AAV Quant application.
Desalting DNA
Invitrogen UltraPure Salmon sperm DNA solution (from ThermoFisher) was diluted to 2 mg/mL in Tris-EDTA, pH 8.0. The Diluted DNA was pipetted manually into a 30 kDa Unfilter 96 or Unfilter 24. Then, the samples were exchanged into nuclease-free water and further increased to three times concentration.
Table 4 lists important experiment setup and parameters. The Nucleic Acid preset exchange parameters were used in the experiment. At a target removal of 50% per well at 60 psi, the buffer exchange protocol was set to 96% total exchange per well.
Table 4. Key buffer exchange parameters used for DNA concentration in 30 kDa Unfilter 96 and Unfilter 24. Wells was run in six replicates. Source: Unchained Labs
| Parameter | DNA settings | |
|---|---|---|
| Unfilter 96 | Unfilter 24 | |
| Target exchange % | 96% | 96% |
| Target volume removed per cycle | 50% | 50% |
| Initial conc. (mg/mL) | 2 | 2 |
| Initial well volume | 450 µL | 3 mL |
| Target final conc. (mg/mL) | 6 | 6 |
| Target final well volume (µL) | 150 | 1000 |
The analysis of concentration before and after exchange and concentration was performed using the UV/Vis application on the Lunatic platform.
Results
LNP buffer exchange and concentration
An exchange of 99.7 ± 0.0% was achieved by setting a target percent exchange of 99% per well, as shown in Table 5. The final volume post concentration procedure was ultrasonically measured as 436.5 µL ± 12.0 µL, at the target of 450 µL per well.
Table 5. LNP solution concentration and % encapsulation before and after buffer exchange and concentration in a 100 kDa Unfilter 24. Source: Unchained Labs
| LNP parameter | Initial | Target final | Actual final |
|---|---|---|---|
| Conc. (µg/mL) | 26.1 | 78.4 | 83.5 ± 2.3 |
| % encapsulation | 98.0 | 98.0 | 96.5 ± 0.1 |
| Volume (µL) | 1345 | 450 | 436.5 ± 12.0 |
| % exchanged | – | 99 | 99.7 ± 0.0 |
The final LNP concentration was measured based on the final volume noted from Big Tuna at 83.5 ± 2.3 µL/mL, with the target concentration expected at 78.4 µL/mL.
A RiboGreen® assay was employed to quantify the percentage encapsulation of LNP before and after the buffer exchange and concentration procedure.
Post exchange, each well was measured in duplicate and the average percentage encapsulation was presented as 96.5 % ± 0.1% compared to 98% before buffer exchange.
After ethanol removal from the LNP solution, concentration targets were reached, and the percentage encapsulation was constant before and after exchange and concentration.
AAV buffer exchange: Unfilter 96
A value of 96% was set as the target percent exchange per well, and 97.0 ± 0.2% exchange was achieved based on the diavolume added to each well (refer to Table 6).
The final volume per well was ultrasonically measured as 149 ± 1 µL, at the target of 150 µL per well. The final AAV concentration was quantified by Stunner at 2.37E13 ± 1.10E12 cp/mL, with the target concentration expected at 2.38E13 cp/mL.
The Stunner was used to quantify the AAV empty/full ratio. The AAV before buffer exchange had an empty/full capsid ratio of 23/77. Post buffer exchange, 28/77 was noted as the value of empty/full ratios.
A small decline in the capsid empty/full ratio was traced, potentially because of the removal of free DNA during the exchange process.
Table 6. AAV concentration and empty/full ratio before and after buffer exchange and concentration in a 30 kDa Unfilter 96. Source: Unchained Labs
| AAV parameter | Initial | Target final | Actual final |
|---|---|---|---|
| Conc. (cp/mL) | 7.89E12 | 2.38E13 | 2.37E13 ± 1.10E12 |
| Well volume (µL) | 450 | 150 | 149 ± 1 |
| % exchanged | – | 96 | 97.0 ± 0.2 |
| Capsid empty/full ratio | 23/77 | 23/77 | 28/72 |
AAV buffer exchange: Unfilter 24
A value of 96% was set as the target percent exchange per well, and 96.2 ± 0.2% exchange was yielded based on the diavolume added to each well, as shown in Table 7. The end result of volume per well was measured at 524 µL ± 23 µL, thus achieving the target of 500 µL per well.
Table 7. AAV concentration and empty/full ratio before and after buffer exchange and concentration in a 30 kDa Unfilter 24. Source: Unchained Labs
| AAV parameter | Initial | Target final | Actual final |
|---|---|---|---|
| Conc. (cp/mL) | 8.17E12 | 2.45E13 | 3.13E13 ± 9.54E11 |
| Well volume | 1.5 mL | 500 µL | 524 ± 23 µL |
| % exchanged | – | 96 | 96.2 ± 0.2 |
| Capsid empty/full ratio | 29/71 | 29/71 | 36/64 |
The Stunner was used to quantify the final AAV concentration at 3.13E13 cp/mL ± 9.54E11 cp/mL, with a target concentration expected at 2.45E13 cp/mL. The Stunner was also used to determine the AAV empty/full ratio.
The AAV before buffer exchange showed an empty/full capsid ratio of 29/71. Post buffer exchange, the empty/full ratio was measured at 36/64. The sample was a little more concentrated than the targeted value.
A minute decline in the capsid empty/full ratio was observed, potentially due to the removal of free DNA during the exchange process.
Dilute AAV: Reduce sample volume
The experiment attempted to concentrate 18 mL of AAV9 to 8 mL using the Reduce Sample Volume application. Furthermore, the reduced volume sample was concentrated in the same Unfilter 24 well plate with the Concentrate Only application down to a 500 µL final volume for 36-fold concentration.
The Reduce Sample Volume application was employed to reduce the 18 mL sample to 7.913 ± 0.022 mL. The same wells were then concentrated with the Concentration Only application and reached a volume of 523.7 µL ± 4.2 µL, compared to the target of 500 µL/well. This is shown in Table 8.
Table 8. AAV sample volume reduced 36-fold in a 30 kDa Unfilter 24. Source: Unchained Labs
| AAV parameter | Initial | Target final | Actual final |
|---|---|---|---|
| Conc. (cp/mL) | 4.82E11 | 1.74E13 | 1.88E13 |
| Well volume | 18 mL | 500 µL | 523.7 ± 4.2 µL |
| Capsid empty/full ratio | 33.3/66.7 | 33.3/66.7 | 24.5/75.5 |
The final AAV concentration was quantified by Stunner at 1.88E13 cp/mL expecting a target concentration at 1.74E13 cp/mL. The AAV before concentrating featured an empty/full capsid ratio of 33.3/66.7. The empty/full ratio was measured at 24.5/75.5 after concentrating.
There were no remarkable discrepancies in the capsid empty/full ratio.
Desalting DNA: Unfilter 96
As represented in Table 9, a target percent exchange of 96% was set per well, achieving an exchange of 97.8% ± 1.4%. The end volume per well was measured ultrasonically as 137 µL ± 9 µL at the target of 150 µL per well.
The final DNA concentration was quantified by Lunatic (from Unchained Labs) as 7.163 ± 0.352 mg/mL, with a target concentration expected at 6.243 mg/mL.
Table 9. Desalting DNA into nuclease-free water in a 30 kDa Unfilter 96. Source: Unchained Labs
| Unfilter 96 | Initial | Target final | Actual final |
|---|---|---|---|
| DNA conc. (mg/mL) | 2.081 | 6.243 | 7.163 ± 0.352 |
| Well volume (µL) | 450 | 150 | 137 ± 9 |
| % exchanged | – | 96 | 97.8 ± 1.4 |
Desalting DNA with Unfilter 24
A value of 96% was set as the target percent exchange per well, achieving an exchange of 98.5% ± 0.2%. This is represented in Table 10. The final volume per well was measured ultrasonically as 1.022 mL ± 0.027 mL, against the target of 1 mL per well.
Table 10. Desalting DNA into nuclease-free water in a 30 kDa Unfilter 24. Source: Unchained Labs
| Unfilter 24 | Initial | Target final | Actual final |
|---|---|---|---|
| DNA conc. (mg/mL) | 2.081 | 6.243 | 6.707 ± 0.204 |
| Well volume (mL) | 3 | 1 | 1.022 ± 0.027 |
| % exchanged | – | 96 | 98.5 ± 0.2 |
The final concentration of DNA was quantified at 6.707 mg/mL ± 0.204 mg/mL, using the Lunatic platform, with a target concentration expected at 6.243 mg/mL, which is somewhat more concentrated than the target.
Conclusion
AAVs, LNPs, nucleic acids and proteins have a highly differential sizes and conformations with different working concentrations and buffer conditions between sample types. Such differences significantly affect the filtration rate and efficiency of buffer exchange and concentration procedures.
Big Tuna’s preset processing parameters modify the UF/DF conditions, such as pressure and sample removal rates, as per the specific molecule type and its concentration. This enables optimal exchange and concentration of all sample types while maintaining sample integrity.
In the case of assembled molecules like AAVs and LNPs, the exchange must be faster and retain the payload integrity. AAVs are exchanged at a lower pressure as they tend to flow more quickly, and the lower pressure ensures they can consitently concentrate to the optimal concentration.
Larger molecules such as LNPs, and higher concentrations of nucleic acids are exchanged under higher pressure and higher exchange rates to enhance the processing speed.
Big Tuna offers the flexibility needed to exchange the larger assemblies along with small proteins and nucleic acids, making it an ideal fit for gene therapy sample preparation laboratories.
About Unchained Labs
Unchained Labs is all about helping biologics researchers break free from tools that just don’t cut it. Unleashing problem-tackling products that make a huge difference in the real science they do every day.
That’s their mantra, their promise and they own it. They live by an unconventional strategy for a start-up: they’re buying commercial businesses and developed technologies, adding their magic touch to turn them into breakthrough products, investing massively in customer-facing teams and then selling those products like gangbusters.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
