To the best of our knowledge, this is the first prospective study demonstrated that two separate predictive equations is not necessary for acute (≤5 days) and late (≥6 days) phases of critical illness. Hence, we have developed and internally validated a single PE and demonstrated that it is useful for estimating the REE of both acute and late phases of critical illness in the Asian population.
Our previous study [14], which compared measured REEs using IC with predicted REEs from 15 commonly used PEs, showed that none of the REEs calculated using those PEs had very good accuracy and agreement at different phases of critical illness. Besides, we also found that patients in the acute phase had significantly lower mean REEs than patients in the late phase [14]. Considering the importance of these variations in energy requirements in different phases of critical illness, the recent ESPEN guidelines recommended a gradual increment of energy provision [2]. These provide us with the rationale to assess metabolic determinants of REE for the acute and late phases of critical illness.
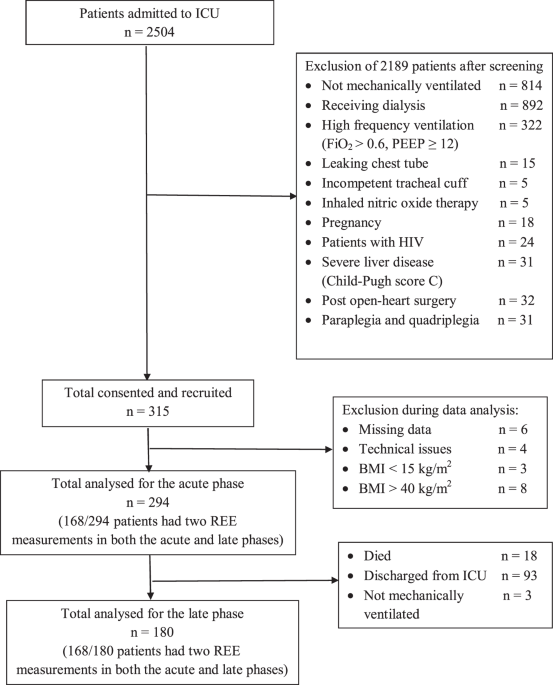
In this study, the predicted REE calculated from two separate PEs developed from patients that had two IC measurements in acute and late phases had mean absolute percentage differences of <20% when compared to measured REE in both phases, even though there appears to be a significant change in the severity of diseases between the two phases. In clinical practice, a 10–20% difference between predicted and measured REE has been considered acceptable. Based on the current literature, a difference of ±20% of energy intake does not produce any clinically meaningful difference in important outcomes [15, 25]. This diminished the clinical reasoning for needing two separate equations for the different phases. A larger difference of measured REE between the acute and late phases may become more evident with a larger sample size and time-lapse. From these results, we decided to develop and validate a single PE among all patients with REE measured in the acute phase only, given the larger sample size. This single PE developed from the acute phase has been found to be optimal for estimating REE in both the acute and late phases.
Studies have shown that REE is influenced by many factors, including age, sex, body composition, body temperature, body movement, environmental temperature, heart rate, and disease status [26,27,28]. Pharmacological agents, such as analgesics, sedatives, and muscle relaxant reduce REE, while vasopressors increase REE [29]. REE can fluctuate throughout illness and can increase due to stress-induced metabolic effects in critically ill patients [30]. In this study, height, weight, minute ventilation and age were the main determinants of REE, and they explained about 44.2% of the variations in REE. The explanatory power of our variables is comparable with those reported in other studies that had developed PEs for ICU populations, such as Penn State [31], Raurich [32], Brandi [33], Faisy [13] and Swinamer [12], where the reported explanatory power ranges from 37% to 77%. The moderate performance of PEs developed for critically ill patients is expected as many factors will influence REE [26,27,28,29,30] and a PE estimates REE from only a few variables that are highly correlated with measured REE.
In the present study, weight and age were the ‘static’ variables selected for use in the prediction models of both the acute and late phases. Older patients may have lower REEs partly because of age-associated changes in body composition and the relative size of fat-free mass (FFM) components [27, 34, 35]. Many studies have shown that body weight and FFM (the metabolizing mass of the body) correlate with REE [35,36,37]. However, the relationship between REE and body weight is nonlinear at the extremes of body weight [22, 27, 38]. A disturbance in the ratio of total body weight, organ, and muscle can distort the association between body weight and REE, especially among underweight, obese and muscular individuals [34, 36]. For these reasons, patients with BMI < 15 kg/m2 and BMI > 40 kg/m2 were excluded from this analysis.
In this study, multivariate analysis showed that the independent variables defining REE were those related to metabolism (age, weight, height, and minute ventilation). Of note, one “dynamic” variable which is minute ventilation was selected in the final prediction model. Minute ventilation, which is determined by the respiratory rate and tidal volume, depends on the sedation level or the ventilator setting. During the acute phase, patients are unstable, and the dynamic nature of minute ventilation may be able to better reflect the metabolic rate of the patients in this phase. Minute ventilation has also been included in previous equations for estimating the REE of critically ill patients [9, 13, 33]. Minute ventilation maintains acid-base homoeostasis and stable carbon dioxide status in an individual. The relationship between minute ventilation and REE is predicted because carbon dioxide production (VCO2) is a part of the Weir equation (27, 28). Furthermore, the increase in REE with an increase of VCO2 is the main cause for increased minute ventilation requirements in long-staying mechanically ventilated patients [39]. Compared with PEs that use only ‘static’ variables, it is preferable to include dynamic variables in the PEs because PEs with “dynamic” variables are more consistent with REEs measured by IC [14].
The present study used predicted body cell mass (BCM) to explore the relationship between measured REE and body composition. This was a significant but weak correlation (r = 0.234 in the acute phase, r = 0.257 in late phase; p < 0.05) and thus was not selected for use in either of the prediction models. The probable reasons were that body composition values are influenced by stress conditions, injury and abnormal fluid status in critical illness [40, 41], and the predictive equation for calculating BCM [42] used in our study may be less accurate. The variable ‘energy intake in the previous 24 h’ was also not selected for use in the prediction model in the late phase even though statistically it is correlated with REE. This is because the problem of multicollinearity might exist as energy intake was guided by measured REE since early phase and use of this variable will lead to large variations of calculated REE as the variations in energy intake of patients can range from 0 kcal/day when nil by mouth to about 2000 kcal/day when given full feeding. Furthermore, the effect of DIT on REE is less than 5% when patients are not overfed and are on continuous nutrition support [43,44,45]. IC measurements in our study were performed without discontinuing nutrition support and patients were not overfed (mean energy adequacy 87.1 ± 20.2%). The current study also did not include body temperature in the new predictive model because most of the patients were in normothermia condition (mean 36.71 ± 0.86 °C) during IC measurements, although variations of 5% REE per degree Celsius has been found in other ICU studies [13, 32].
Recently, ventilator-derived VCO2 has been proposed for estimating REE as it was shown to be more accurate than PEs [46, 47]. However, further validation showed that it has a low agreement with IC-measured REE [48]. Experts have also suggested that this simplistic approach cannot reflect the complex physiological changes that critically ill patients undergo [49]. Besides, this method needs a special ventilator that can measure VCO2. Thus, PEs are still routinely used in daily practice and in critical care nutrition trials when IC is not feasible [15, 24].
This study has several limitations. First, the PEs generated in this single-centre study has limited generalizability. Second, as the measured or estimated body weight may not reflect actual weight (due to acute fluid shifts), this could have introduced errors in the prediction model. Third, despite efforts to ensure that every measurement was done in a standardized manner, the accuracy of IC measurements was inevitably influenced by metabolic factors, such as changes in body composition, medications, disease status, changes of ventilator mode, nutrients absorption and body temperature.
On the other hand, this study also has several strengths. First, the PE for critically ill Asian patients were developed and validated by considering the variable, dynamic, and complex nature of metabolic changes at different phases of critical illness. Second, this is a prospective study with a relatively large sample size and large number of variables in both the acute and late phases. Third, the use of multi-fold cross-validation is considered a robust internal-external validation method for the PEs, which can accurately predict out-of-sample accuracy and use data more efficiently as all observations are used for both testing and training [20, 21]. Fourth, the use of a single carefully calibrated device and the application of the standardized method for measuring REE minimized variations in the measurements. Lastly, a Microsoft Excel Tah et al. equation calculator (Supplementary File), which is a practical and simple-to-use tool is provided to facilitate the use of the developed PE in the ICU setting.