Healthy skin is determined by a complex arrangement and interaction of a number of factors. This case study presents a clinical trial that aimed to evaluate an oral supplement’s effect on some of these skin dimensions.
Challenges and objectives
The experience that the Atlantia team offered when designing and performing skin studies to support a health claim was a controlling factor in the success of this partnership.
The main objective of the clinical trial was to establish the impact on Trans-Epidermal Water-Loss (TEWL) following 12-week daily consumption of an oral supplement in contrast to a placebo.
Other key objectives of the trial were to determine:
- Omega-3-index
- Skin firmness
- Skin hydration
- Skin Lipid content
- Subjective evaluation of Skin quality, hydration, moisture and elasticity
How Atlantia’s solution helped
Atlantia’s team is trained and skilled in evaluating skin indications using specific study equipment. Their experience and expertise in designing and performing studies to ICH-GCP standards were major influences and factors in this partnership.
Image Credit: Atlantia Clinical Trials

Image Credit: Atlantia Clinical Trials
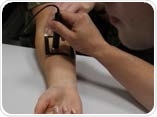
Image Credit: Atlantia Clinical Trials
About the study
The study was a randomized, double-blinded, parallel, placebo-controlled clinical trial which drew from a healthy adult population. The study necessitated visits over a 15-week period to conduct a 12-week intervention period while obtaining study objectives at each participant’s visit.
Trans-Epidermal Water Loss (skin barrier function) was evaluated using TEWAMeter®, skin firmness using Cutometer®, skin hydration using Corneometer® and Omega3-index using a finger-prick blood test.
Subjects were subjected to a screening process in order to identify eligible participants. All participants were randomized. Study subjects orally consumed one capsule of investigational product each morning of the intervention period.
Image Credit: Atlantia Clinical Trials
Participants completed the consenting process and were appropriately informed to provide written consent prior to the screening visit. Participants underwent a screening visit to assess their eligibility for the study, and if all eligibility requirements were met, they were scheduled for a follow-up visit.
At subsequent visits, participants were further evaluated for eligibility, and if each of the inclusion/exclusion criteria were satisfied, baseline assessments were conducted, and randomization of the participants into one of two treatment groups was performed to a ratio of 1:1.
After 12 weeks of supplementation of the investigational supplement, fascinating and intriguing findings were seen concerning an improvement in TEWL and Omega-3 Index blood spot levels when compared to the placebo.
While there were anomalies in the data, this is not atypical given the wide range of factors that can influence TEWL. Further investigation may offer further insight and information on these findings.
About Atlantia Clinical Trials
Atlantia Clinical Trials Ltd is a CRO that specializes in conducting studies in foods, beverages and supplements for companies world-wide that want to scientifically validate their functional ingredients to support an: EFSA (European Food Safety Authority) Health Claim; FDA (Food & Drug Administration) Structure Function Claim; or General Product Marketing Claim.
Atlantia works with world leading scientists (among the top cited 1% internationally, in the areas of digestive health and functional foods) at the: APC Microbiome Institute in University College Cork, Ireland; Teagasc, Moorepark, Ireland and recognized centers of excellence globally.
Atlantia runs and operates its own clinic sites and conducts all studies to ICH-GCP standard (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use – Good Clinical Practice). Its team includes physician experts in digestive health, mental health (psychological stress and cognition), cardiovascular health, sports performance, metabolic disease, bone health, immune health and healthy ageing. The clinical team also includes project managers, research nurses, nutritionists, certified sports trainers and lab researchers.
Atlantia manages all elements from protocol design, placebo manufacture, recruitment, and study execution, to sample and data analysis, statistics and report/dossier preparation to provide a service which is technically, scientifically and clinically superior.
The clinical studies cover a broad spectrum of functional food and beverage categories, such as dairy, cereal, probiotic, different protein forms, infant-specific foods, vitamins/minerals, plant or marine extracts and medical foods.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
